马上注册,结交更多好友,享用更多功能,让你轻松玩转社区。
您需要 登录 才可以下载或查看,没有账号?注册
×
<p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family: 宋体;color:#222222">用于处理高硅的铝土矿,将铝土矿、碳酸钠和石灰按一定比例混合配料,在回转窑内烧结成由铝酸钠</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">(Na2O·Al2O3)</span><span style="font-size:15px;font-family:宋体;color:#222222">、铁酸钠</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">(Na2O·Fe2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">、原硅酸钙(</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2CaO·SiO2</span><span style="font-size: 15px;font-family:宋体;color:#222222">)和钛酸钠(</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">CaO·TiO2</span><span style="font-size: 15px;font-family:宋体;color:#222222">)成的熟料。然后用稀碱溶液溶出熟料中的铝酸钠。此时铁酸钠水解得到的</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">NaOH</span><span style="font-size:15px;font-family:宋体;color:#222222">也进入溶液。如果溶出条件控制适当,原硅酸钙就不会大量地与铝酸钠溶液发生反应,而与钛酸钙、</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Fe2O3·H2O </span><span style="font-size:15px;font-family:宋体;color:#222222">等组成赤泥排出。溶出熟料得到的铝酸钠溶液经过专门的脱硅过程,</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">SiO2O</span><span style="font-size:15px;font-family:宋体;color:#222222">形成水合铝硅酸钠</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">(</span><span style="font-size:15px;font-family:宋体;color:#222222">称为钠硅渣</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">)</span><span style="font-size:15px;font-family:宋体;color:#222222">或水化石榴石</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">3CaO·Al2O3·xSiO2·(6</span><span style="font-size:15px;font-family:宋体;color:#222222">-</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2x)H2O</span><span style="font-size: 15px;font-family:宋体;color:#222222">沉淀</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">(</span><span style="font-size:15px;font-family:宋体;color:#222222">其中</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">x≈0.1)</span><span style="font-size: 15px;font-family:宋体;color:#222222">,使溶液提纯。把</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">CO2</span><span style="font-size:15px;font-family:宋体;color:#222222">气体通入精制铝酸钠溶液,和加入晶种搅拌,得到氢氧化铝沉淀物和主要成分是碳酸钠的母液。氢氧化铝经煅烧成为氧化铝成品。水化石榴石中的</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Al2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">可以再用含</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Na2CO3</span><span style="font-size: 15px;font-family:宋体;color:#222222">母液提取回收。</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family: 宋体;color:#222222">碱石灰烧结法的主要化学反应如下:</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family: 宋体;color:#222222">烧结:</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Al2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Na2CO3─→Na2O·Al2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">CO2</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Fe2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Na2CO3─→Na2O·Fe2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">CO2</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">SiO2</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2CaCO3─→2CaO·SiO2</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2CO2</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">TiO2</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">CaCO3─→CaO·TiO2</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">CO2</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family: 宋体;color:#222222">熟料溶出:</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Na2O·Al2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">4H2O─→2NaAl(OH)4</span><span style="font-size:15px;font-family:宋体;color:#222222">(溶解)</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Na2O·Fe2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2H2O─→Fe2O3·H2O↓</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2NaOH</span><span style="font-size:15px;font-family:宋体;color:#222222">(水解)</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family: 宋体;color:#222222">脱硅:</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">1.7 Na2SiO3</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2NaAl(OH)4─→Na2O·Al2O3·1.7SiO2·nH2O↓</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">3.4NaOH</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">3 Ca(OH)2</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2NaAl(OH)4</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">x Na2SiO3─→ 3CaO·Al2O3·x SiO2·(6</span><span style="font-size:15px;font-family:宋体;color:#222222">-</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">x)H2O↓</span><span style="font-size: 15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2(1</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">x)NaOH</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family: 宋体;color:#222222">分解:</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">2NaOH</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">CO2─→Na2CO3</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">H2O</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">NaAl(OH)4─→Al(OH)3↓</span><span style="font-size:15px;font-family:宋体;color:#222222">+</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">NaOH</span></p><p style="margin-top:auto;margin-bottom: auto;text-align:left;line-height:25px;background: white"><span style="font-size:15px;font-family: 宋体;color:#222222">中国碱石灰烧结法生产氧化铝的主要技术成就是:在熟料烧成中采用低碱比配方,在熟料溶出工艺中采用二段磨料和低分子比溶液,以抑制溶出时的副反应损失,使熟料中</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Na2O</span><span style="font-size:15px;font-family:宋体;color:#222222">和</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Al2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">的溶出率分别达到</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">94</span><span style="font-size:15px;font-family:宋体;color:#222222">~</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">96</span><span style="font-size:15px;font-family:宋体;color:#222222">%和</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">92</span><span style="font-size:15px;font-family:宋体;color:#222222">~</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">94</span><span style="font-size:15px;font-family:宋体;color:#222222">%。</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Al2O3</span><span style="font-size:15px;font-family:宋体;color:#222222">的总回收率约</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">90</span><span style="font-size:15px;font-family:宋体;color:#222222">%,每吨氧化铝的</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">Na2CO3</span><span style="font-size: 15px;font-family:宋体;color:#222222">的消耗量约</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">95</span><span style="font-size:15px;font-family:宋体;color:#222222">公斤。碱石灰烧结法可以处理拜耳法不能经济地利用的低品位矿石,其铝硅比可低至</span><span style="font-size:15px;font-family:'Arial','sans-serif';color:#222222">3.5</span><span style="font-size:15px;font-family:宋体;color:#222222">,原料的综合利用较好,有其特色。</span></p><p><br/></p> |
 FL3322 压力传感器读取器用于 TS633 PLC191 人气#I/O
FL3322 压力传感器读取器用于 TS633 PLC191 人气#I/O DA200-N伺服面板查看故障记录报警代码是Er2689 人气#通用伺服系统
DA200-N伺服面板查看故障记录报警代码是Er2689 人气#通用伺服系统 关于DA200-N通过总线驱动器通过参数0x4000644 人气#通用伺服系统
关于DA200-N通过总线驱动器通过参数0x4000644 人气#通用伺服系统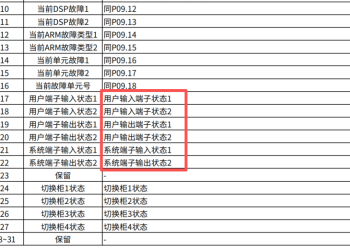 GD5000 profibus DP通信报文给定值和实际值2302 人气#高压变频器
GD5000 profibus DP通信报文给定值和实际值2302 人气#高压变频器