马上注册,结交更多好友,享用更多功能,让你轻松玩转社区。
您需要 登录 才可以下载或查看,没有账号?注册
×
<p style="margin-top:0;margin-right:0;margin-bottom:9px;margin-left:0;line-height:16px;background:white"><span style="font-size:9px;font-family: '微软雅黑','sans-serif';color:#333333">电解氧化铝(下面是公式):</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size: 14px;font-family:'微软雅黑','sans-serif';color:#333333">2Al<sub>2</sub>O<sub>3 = </sub></span><sup><span style="font-size:14px;font-family:'微软雅黑','sans-serif';color:#333333">通电</span></sup><span style="font-size:14px;font-family:'微软雅黑','sans-serif';color:#333333"> <sub>== </sub>4 Al +3O<sub>2</sub></span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">工业氧化铝是由</span><strong><span style="font-size:9px;font-family: '微软雅黑','sans-serif';color:red">铝矾土(Al</span></strong><strong><span style="font-size:9px;font-family:'Cambria Math','serif';color:red">₂</span></strong><strong><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:red">O</span></strong><strong><span style="font-size:9px;font-family:'Cambria Math','serif';color:red">₃</span></strong><strong><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:red">·</span></strong><strong><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:red">3H</span></strong><strong><span style="font-size:9px;font-family:'Cambria Math','serif';color:red">₂</span></strong><strong><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:red">O</span></strong><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">)和硬水铝石制备的,对于纯度要求高的Al</span><span style="font-size:9px;font-family: 'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family: '微软雅黑','sans-serif';color:#333333">O</span><span style="font-size: 9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">,一般用化学方法制备。</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">有许多同质异晶体,目前已知的有</span><span style="font-size: 9px;font-family:'微软雅黑','sans-serif';color:#333333">10</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">多种,主要有3种晶型,即α-Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">、β</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">-Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">、γ</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">-Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">。其中结构不同性质也不同,在</span><span style="font-size: 9px;font-family:'微软雅黑','sans-serif';color:#333333">1300</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">℃以上的高温时几乎完全转化为α-Al</span><span style="font-size:9px;font-family: 'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family: '微软雅黑','sans-serif';color:#333333">O</span><span style="font-size: 9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">。</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;background:white"><a href="https://iknow-pic.cdn.bcebos.com/9f510fb30f2442a7c18d0b71dc43ad4bd0130266" title=""点击查看大图" t "><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#3F88BF;text-underline:none"><img width="600" height="376" src="http://luntan.szhuitianxia.com/pc/ueditor/themes/default/images/spacer.gif" alt="https://iknow-pic.cdn.bcebos.com/9f510fb30f2442a7c18d0b71dc43ad4bd0130266?x-bce-process=image%2Fresize%2Cm_lfit%2Cw_600%2Ch_800%2Climit_1%2Fquality%2Cq_85%2Fformat%2Cf_auto" style="background:url(http://luntan.szhuitianxia.com/pc/ueditor/lang/zh-cn/images/localimage.png) no-repeat center center;border:1px solid #ddd"/></span></a></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><strong><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">扩展资料:</span></strong></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">和酸反应:</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size: 9px;font-family:'微软雅黑','sans-serif';color:#333333">Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333"> + 6HCl = 2AlCl</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333"> + 3H</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size: 9px;font-family:'微软雅黑','sans-serif';color:#333333">Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333"> + 6H</span><span style="font-size:9px;font-family:'MS Gothic';color:#333333">⁺</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333"> = 2Al</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">³</span><span style="font-size:9px;font-family:'MS Gothic';color:#333333">⁺</span><span style="font-size:9px;font-family: '微软雅黑','sans-serif';color:#333333"> + 3H</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">和熔融的碱反应:</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size: 9px;font-family:'微软雅黑','sans-serif';color:#333333">Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333"> + 2NaOH== 2NaAlO</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">(偏铝酸钠)</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333"> + H</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">和碱溶液反应:</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size: 9px;font-family:'微软雅黑','sans-serif';color:#333333">Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">+ 2NaOH +3H</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O = 2Na[Al(OH)</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₄</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">]</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">(四羟基合铝酸钠)</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;line-height:16px;background:white"><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">也可以简写为:Al</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₃</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">+2OH</span><span style="font-size:9px;font-family:'MS Gothic';color:#333333">⁻</span><span style="font-size:9px;font-family: '微软雅黑','sans-serif';color:#333333">=2AlO</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂⁻</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">(偏铝酸根离子)</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">+H</span><span style="font-size:9px;font-family:'Cambria Math','serif';color:#333333">₂</span><span style="font-size:9px;font-family:'微软雅黑','sans-serif';color:#333333">O</span></p><p style="margin-top:9px;margin-right:0;margin-bottom:9px;margin-left: 0;background:white"><br/></p><p><br/></p> |
 FL3322 压力传感器读取器用于 TS633 PLC519 人气#I/O
FL3322 压力传感器读取器用于 TS633 PLC519 人气#I/O DA200-N伺服面板查看故障记录报警代码是Er2950 人气#通用伺服系统
DA200-N伺服面板查看故障记录报警代码是Er2950 人气#通用伺服系统 关于DA200-N通过总线驱动器通过参数0x4000881 人气#通用伺服系统
关于DA200-N通过总线驱动器通过参数0x4000881 人气#通用伺服系统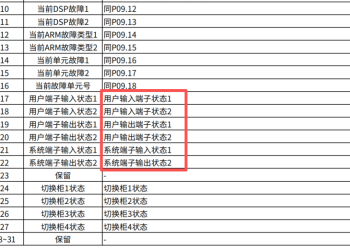 GD5000 profibus DP通信报文给定值和实际值6762 人气#高压变频器
GD5000 profibus DP通信报文给定值和实际值6762 人气#高压变频器